Aduhelm is the brand name under which the Alzheimer's drug Aducanumab is marketed. It is developed by the U.S. company Biogen. The drug is to be administered as an intra-venous infusion every four weeks.
The Food and Drug Administration (FDA) approved Aducanumab in June 2021, making it the first Alzheimer's disease medication approved by the FDA since 2003. It is also the first ever approved Alzheimer's drug targeting the presence of amyloid beta (a type of protein) plaques in the brain.
However, almost everything about this drug is controversial, from the theory on which the drug is developed, to its clinical trials data, to the FDA approval itself. This article aims to distill the complicated information down to a few points, which might help patients make more informed decisions.
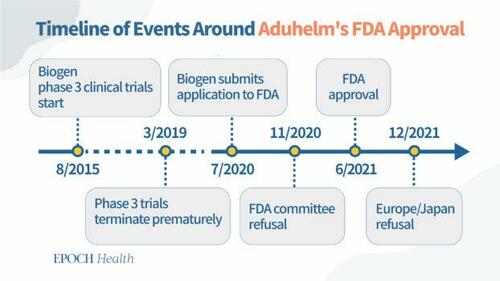
Key Dates Surrounding the FDA Approval of Aducanumab
July 2020: Biogen completes submission of aducanumab data (from previously discontinued clinical trials) to FDA.
November 2020: FDA external advisory committee rejects aducanumab because the data failed to prove the drug's efficacy in reducing cognitive decline in Alzheimer's patients.
June 2021: FDA grants accelerated approval for aducanumab for patients of all stages of Alzheimer's disease, on the condition that Biogen conducts post-approval trials (phase 4 trials) to verify the efficacy of the drug.
Biogen's Troubled Clinical Trials
Biogen started two practically identical phase 3 clinical trials in August 2015 to evaluate the safety and efficacy of different aducanumab doses in patients with early stage Alzheimer's disease.
But in March 2019, Biogen terminated the trials after an interim futility analysis, run by an independent data-monitoring committee, predicted that the trials were unlikely to meet their primary outcomes.
Only seven months later, in a surprise press release, Biogen revoked its earlier decision citing that a new analysis with another three months of data produced positive results. The company stated, "The positive results of this new analysis were driven primarily by greater exposure to high dose aducanumab in the larger dataset as compared to data available at the time of the futility analysis." In the same press release, Biogen announced its plan to apply for FDA approval, "based on discussions with the FDA, the Company plans to submit a Biologics License Application in early 2020."
FDA Granted Approval Despite Experts' Disapproval
It is routine operation for FDA to invite subject matter experts, who are "outside the government with minimal conflicts of interest" to form external advisory committees and give non-binding recommendations for FDA's consideration. In November 2020, the Peripheral and Central Nervous System Drugs Advisory Committee voted almost unanimously against the approval of Aducanumab. Out of the 11 members in the committee, 10 voted against the approval and one was uncertain.
Seven months later in June 2021, FDA granted accelerated approval to aducanumab as a treatment for patients of all stages of Alzheimer's disease. According to FDA, accelerated approval "allows drugs for serious conditions that filled an unmet medical need to be approved based on a surrogate endpoint." The "surrogate endpoint" for aducanumab was the reduction in the amyloid-beta plaques.
Dr. Patrizia Cavazzoni, Director of FDA Center for Drug Evaluation and Research, wrote in a post-approval statement that the clinical trials data show a reduction in the amyloid-beta plaques, which "is expected to lead to a reduction in the clinical decline of this devastating form of dementia." Cavazzoni also acknowledged that "the data included in the applicant's submission were highly complex and left residual uncertainties regarding clinical benefit."
In other words, although Biogen's clinical trials data failed to prove the drug can slow down cognitive decline in patients, it did show the reduction of amyloid-beta plaque. The FDA awarded speedy approval to the drug based on the assumption that reduction of amyloid-beta plaque "is expected to lead to" a slowdown in cognitive decline in Alzheimer's patients.
Along with the approval, FDA gave Biogen another opportunity to prove the assumption is true via post-approval trials (phase 4 trials), which is due by February 2030. That is to say, Aduhelm will have been on the market for nine years before proof of its efficacy is required. If the drug maker fails to show the clinical benefit nine years after the accelerated approval, FDA might remove the drug from the market.
One more detail about the FDA approval is that the drug was initially approved to treat patients with all stages of Alzheimer's disease. One month after the approval, the FDA limited its treatment to patients with early stage Alzheimer's disease only.
FDA Advisory Committee Members Resign
Within the first week of the FDA approval, three members of the Peripheral and Central Nervous System Drugs Advisory Committee resigned. They were Dr. Aaron Kesselheim at Harvard Medical School, neurologists David Knopman of the Mayo Clinic in Minnesota and Joel Perlmutter of Washington University in St. Louis.
In his resignation letter, Kesselheim called the FDA move "probably the worst drug approval decision in recent U.S. history." Kesselheim also wrote on Tweeter, "Accelerated Approval is not supposed to be the backup that you use when your clinical trial data are not good enough for regular approval."
Accelerated Approval is not supposed to be the backup that you use when your clinical trial data are not good enough for regular approval.
— Aaron Kesselheim (@akesselheim) June 7, 2021
Knopman and Perlmutter published a comment in Neurology in July 2021. They wrote, "the clinical benefit amounted to about 3 months' worth of delay in decline over a year," referring to the "positive results" in Biogen's press release in October 2019. They stated, "Combining the results from the 2 trials found no statistically significant clinical benefit for high-dose aducanumab."
Side Effects and Safety Issues
Brain swelling and brain bleeding are known to be possible side effects of Aduhelm. Biogen conducted two phase 3 studies prior to the FDA approval. The phase 3 studies started in August 2015, but Biogen pulled the plug on both studies three and a half years later, in March 2019. The decision was based on a futility analysis conducted by an independent data monitoring committee, which indicated the trials were unlikely to meet their primary endpoint upon completion. That is, the trials were unlikely to prove the drug's efficacy in slowing cognitive decline in Alzheimer's patients.
The safety data from the terminated phase 3 trials were published in JAMA Neurology in November 2021. The study focused on amyloid-related imaging abnormalities (ARIA), which can cause headache, confusion, dizziness, nausea, brain bleeding, and swelling.
The data showed that 425 out of 1,029 patients, or 41 percent, who received the high dose of the drug-the dose that the FDA later approved-experienced either brain swelling or bleeding. Furthermore, 64 patients had to stop participating in the trials because of brain swelling or bleeding.
In September 2021, months after approval, a 75-year-old woman, a clinical trial patient who lived in Canada, experienced brain swelling after receiving infusions of the drug and died a few days later.
The Amyloid-beta Hypothesis
The theory behind the Aduhelm and a slew of other experimental medications for Alzheimer's disease is the hypothesis that the excessive amount of amyloid-beta plaque in the brain impairs the normal communication within the brain and thus causes cognitive decline.
In fact, the disease is named after the German pathologist Alois Alzheimer, who discovered amyloid-beta plaque in the brain of a patient.
But this hypothesis has not been proven and is by itself controversial.
Three-Quarters of FDA Funding Is From Drug Makers
The Project On Government Oversight (POGO) is a nonpartisan independent watchdog that investigates and exposes waste, corruption, abuse of power, and when the government fails to serve the public or silences those who report wrongdoing.
A two-part investigative report by POGO details the fact that the FDA is in the pocket of the industry it regulates. The report says, "the agency, whose responsibilities include making sure that prescription drugs sold in the United States are safe and effective, receives almost three-quarters of its funding for that work from drug makers."
Part II of the report exposes the "voice" of the patients at FDA meetings is often funded by drug companies as well.
European and Japanese Agencies Reject Aduhelm
In October 2020, Biogen applied for market permit in Europe. In December 2021, six months after FDA's accelerated approval of Aduhelm, the European Medicines Agency rejected Biogen's application to market Aduhelm in Europe.
The EMA considered "the benefits of Aduhelm did not outweigh its risks." First, the clinical trial results "were conflicting and did not convincingly show that Aduhelm was effective at treating adults with early-stage Alzheimer's disease." Additionally, brain scans of some clinical trial patients suggest swelling or bleeding in the brain, which is a significant safety concern.
In the same month, Japan's Committee on New Drugs also rejected Biogen's application and requested more data to prove the drug's efficacy.
via zerohedge